Description
(These are general health-support benefits permitted for dietary supplements. No disease claims made.)
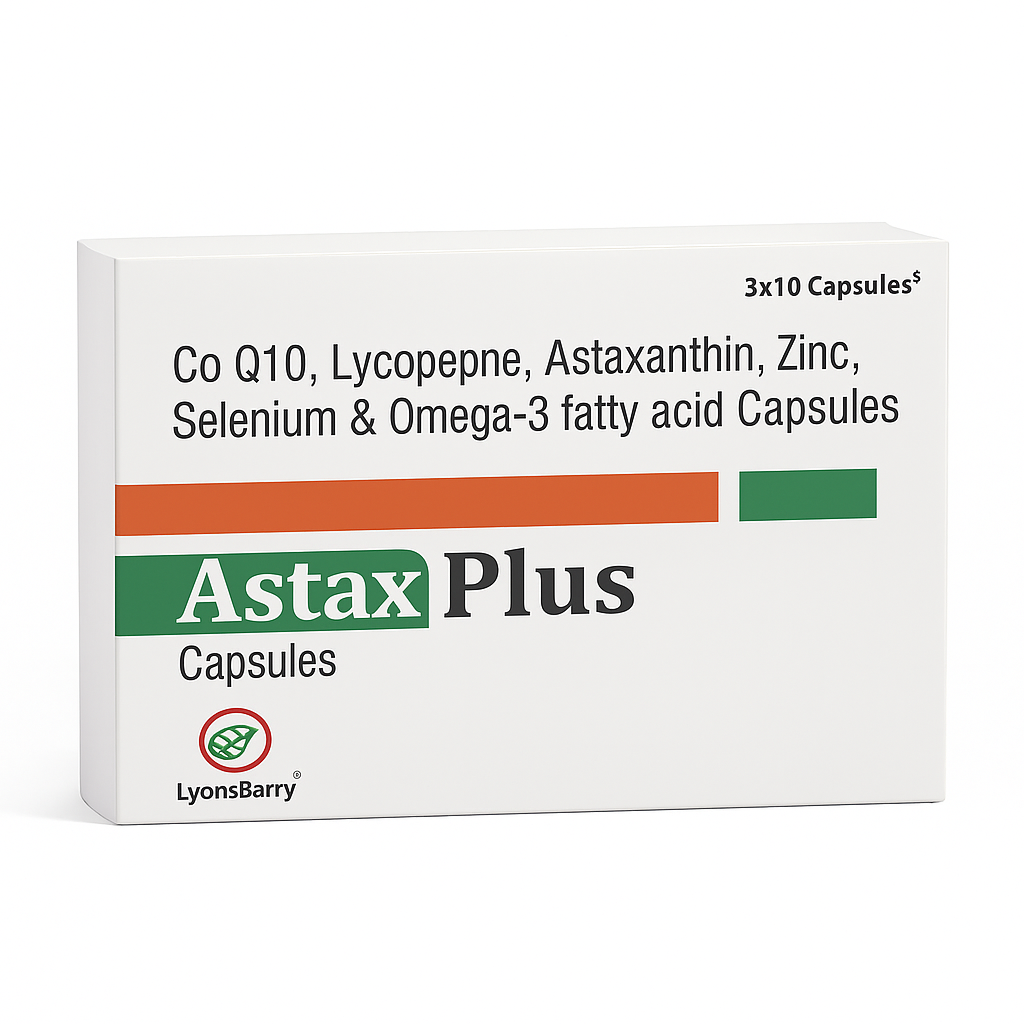
Astax Plus is a scientifically formulated premium dietary antioxidant supplement designed to support cellular energy, immune health, cardiovascular function, skin wellness, and overall vitality. Developed under stringent quality standards, the formula combines clinically researched bioactive, including Coenzyme Q10, Lycopene, Astaxanthin, Zinc, Selenium, and Omega-3 fatty acids, offering synergistic protection against oxidative stress and free-radical damage.
Ideal for adults seeking enhanced cellular support, heart & vascular wellness, anti-oxidative protection, and nutritional balance especially individuals under stress, older adults, athletes, and those recovering from fatigue.
Recommended Usage
Dosage: As directed by a licensed Dietician or Healthcare Professional.
Storage Conditions:
Store below 25°C in a cool, dark, dry place. Keep away from heat, moisture, and direct sunlight.
Safety: Keep out of reach of children. Do not use if capsule is damaged.
Regulatory & Compliance Information
Category: Dietary Food Supplement / Nutraceutical Capsules
Not for Medicinal Use
US FDA Registered : 19184393838
FSSAI Lic. No.: 10822999000088
Batch No.: CLB-0345
Manufacturing Date: 09-2025
Expiry Date: 02-2027
Packaging Format: 3 × 10 Blister Capsules
Country of Origin: Made in India
Marketer: LyonsBarry®, Ballia Corpus – NGM Taraori, Karnal, Haryana, India
Manufacturer: Lyonsbarry®, Karnal, Haryana, India
Export Standard Certifications: GMP | ISO Certified facility
US FDA Registered : 19184393838
International Quality Assurance
Manufactured in accordance with GMP (Good Manufacturing Practices) & ISO-compliant standards.
Process and packaging meet international guidelines for nutraceutical products.
Tested for purity, safety, and stability with documented traceability & overages to compensate storage loss.
QR Verification link included for authenticity.
Suitable for international distribution including NAFTA, EU, GCC, WANA, ASEAN, CIS, Africa, South Asia, and Oceania regions under nutraceutical / food supplement category.
Ideal retail sectors:
Pharmaceutical distributors / OTC pharmacies
Nutrition & wellness stores
Sports nutrition suppliers
Hospitals & dietician-recommended formulas
E-commerce & private label export trade
LABEL ARTWORK COMPLIANCE DECLARATION
The product labelling complies with:
USA: DSHEA / FDA dietary supplement labelling requirements
EU: EFSA Food Supplement Directive
GCC: SFDA / UAE MOH nutraceutical labelling requirements
India: FSSAI Nutraceutical Standard Regulation 2016
Testing Reports: (Sign Up / Login Required for Complete Report for Registration & Import Purposes)
Certificate of Analysis
Heavy metal Testing
Stability Data
Radiation Certificate
Free Sale Certificate